Biography
I obtained a Maters in Biochemistry at the University of Bath in 2006 (placements at Cambridge University & Duke University, USA), followed by a PhD at the University of Bath in neuropsychopharmacology in 2011.
Throughout my post-doctoral training (Schools of Medicine & Psychology, Cardiff University) and Research Fellowship (NMHRI, Cardiff University), I developed an extensive repertoire of research interests in behavioural and molecular neuroscience. I have predominantly utilised preclinical, genetic models of psychiatric illness to provide translatable, clinical insight into debilitating human psychiatric illnesses such as ADHD (Trent et al., 2012), autism (Trent et al., 2014) and schizophrenia (Hall, Trent et al., 2015). I have leveraged behavioural neuroscience techniques, particularly learning and memory in rodents, and complementary molecular, pharmacological and genetic techniques (including RNA sequencing), allowing me to interrogate critical, plasticity- and disorder-relevant neurobiological pathways. I have published primary research and reviews in areas of hippocampal memory (Trent et al., 2015, Clifton et al., 2017), genomics (Hall, Trent et al., 2015) and neuropharmacology (Trent et al., 2009).
Research and scholarship
How do synaptic ‘risk genes’ contribute towards memory and psychiatric illness in people?
Genomic studies have uncovered highly penetrant genetic alterations in schizophrenia and Autism Spectrum Disorder patients. Excitingly, these genes encode synaptic proteins that converge onto a coherent neurobiological pathway including the complex of Fragile X Syndrome protein (FMRP) with cytoplasmic FMRP interacting protein 1 (CYFIP1) and Activity-regulated cytoskeleton-associated protein (ARC), itself under the control of the CYFIP1-FMRP complex (see diagram).
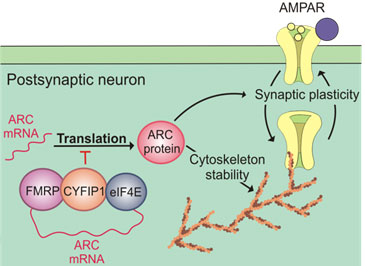 Arc is an activity-dependent, immediate early gene that rapidly accumulates at the synapses upon synaptic activation. Arc protein influences many forms of synaptic plasticity, including LTP and LTD, through AMPA receptor trafficking and stabilisation of the actin cytoskeleton.
Arc is an activity-dependent, immediate early gene that rapidly accumulates at the synapses upon synaptic activation. Arc protein influences many forms of synaptic plasticity, including LTP and LTD, through AMPA receptor trafficking and stabilisation of the actin cytoskeleton.
I have shown the requirement of Arc, as well as AMPA receptors, in regulating memory consolidation and extinction (Trent et al., 2015, 2017) and more recently, the requirement of psychiatric-relevant Cyfip1 protein alongside Arc protein, in mediating extinction of memories (Trent et al., in prep).
In a Cyfip1 genetically-deleted rodent, which precisely mirrors increased genetic risk for psychiatric disorders (equivalent to 15q11.2 deletion patients) the following studies are currently being conducted:
- Next-generation RNA sequencing of hippocampal subfields (Dr N Clifton, NMHRI, Cardiff University)
- Morphological assessment of hippocampal dendrites and spines using DiOlistic labelling techniques (Cardiff University)
- Measures of white matter changes using DTI and myelin thinning (Silva et al., 2019, Nature Communications)
- Molecular and electrophysiological assessment of inhibitory GABAergic function/signalling (Trent et al., 2019a, eNeuro)
I am also interested in synaptic proteins such as activity-regulated Homer1a, both in the context of synaptic plasticity and memory (Clifton et al., 2017) and their likely role in psychiatric disorders (Trent et al., 2019b).
Key aims for future research:
1. Extend my Arc research into fields of Fragile X Syndrome and protein-protein complexes.
2. Explore the role of Arc in a model of Angelman Syndrome, a severe psychiatric disorder caused by the loss of the Arc-degradation protein Ube3a.
3. Understand the role of Arc and Ube3a in the different developmental trajectories of hippocampal memory and infantile amnesia.
References
- Trent S et al., (2009) Chronic treatment with 13-cis-retinoic acid changes aggressive behaviours in the resident-intruder paradigm in rats. European Neuropsychopharmacology. 19(12):876-86
- Trent S et al. (2012) Altered serotonergic function may partially account for behavioral endophenotypes in steroid sulfatase-deficient mice. Neuropsychopharmacology. 37(5):1267-74
- Trent S et al. (2014) Altered brain gene expression but not steroid biochemistry in a genetic mouse model of neurodevelopmental disorder. Molecular Autism. 5(1):21.
- Hall J, Trent S, Thomas KL, O'Donovan MC, Owen MJ (2015) Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biological Psychiatry. 77(1):52-8.
- Trent S et al. (2015) Rescue of long-term memory after reconsolidation blockade. Nature Communications. 6:7897
- Trent S et al. (2017) AMPA receptors control fear extinction through an Arc-dependent mechanism. Learn Mem 24(8):375-380
- Clifton NE, Cameron D, Trent S, Sykes LH, Thomas KL, Hall J (2017) Hippocampal regulation of postsynaptic density Homer1 by associative learning Neural Plast 2017:5959182
- Trent S et al. (2019a) Cyfip1 haploinsufficiency does not alter GABAA receptor δ-subunit expression and tonic inhibition in dentate gyrus PV+ interneurons and granule cells eNeuro 6(3)
- Trent S et al. (2019b) Regulation and function of activity-dependent Homer in synaptic plasticity Molecular Neuropsychiatry 5(3):147-161
- Silva A, Haddon J, Syed Y, Trent S et al., (2019) Cyfip1 haploinsufficient rats show white matter changes, myelin thinning, abnormal oligodendrocytes and behavioural inflexibility Nature Communications 10(1):3455
Publications
School of Life Sciences,
Huxley Building,
Keele University,
Staffordshire,
ST5 5BG
Tel: +44 (0) 1782 734414
Enquiries:
Tel: +44 (0) 1782 734414
Email: lifesciences.office@keele.ac.uk


