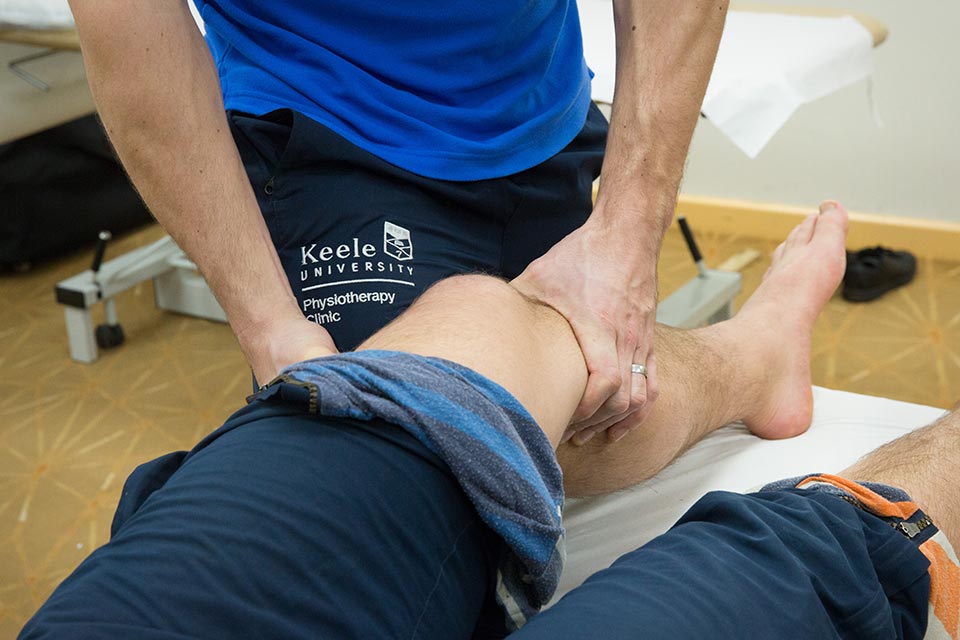
School of Allied Health Professions and Pharmacy
The School of Allied Health Professions and Pharmacy offers a dynamic, forward-thinking, and inclusive learning environment. The School is consistently ranked highly in the Complete University Guide, the Sunday Times, and the Guardian league tables.
Our staff involved in the delivery of education are committed to providing a rich educational experience for all students in a friendly and supportive environment.
Our school has a long-established research programme in pharmacy and physiotherapy related areas.
We offer all patients the option of virtual online or face to face clinic appointments for all your physiotherapy and rehabilitation needs.
Study with us
We have a committed and dedicated team that maintain excellent staff/student relationships, ensuring your get the support you need.
School address:
School of Allied Health Professions and Pharmacy
MacKay Building
Hornbeam Building
Keele University
Staffordshire
ST5 5BG
Enquiries:
Placements team: sahp.practiceplacements@keele.ac.uk
Postgraduate course admin team: sahp.postgraduate-admin@keele.ac.uk
Undergraduate course admin team: sahp.admin@keele.ac.uk
Undergraduate enquiries:
Email: enquiries@keele.ac.uk
Tel: +44 (0)1782 734010
Pharmacy postgraduate enquiries:
Please contact the CPD4ALL team: cpd4all@keele.ac.uk
Pharmacy email: phab.postgraduate@keele.ac.uk
Keele Centre for Medicines Optimisation (KCMO)
Tel: +44 (0)1782 733831 / 734131
The Virtual Patient project enquiries:
Contact our Digital Development team:
Email: pharmacy.digital@keele.ac.uk