Should you do an IPD meta-analysis project?
Systematic reviews are the cornerstone of evidence synthesis and evidence-based decision-making in healthcare. They use transparent methods to identify, appraise and combine a body of research evidence, with the goal of producing summary results that guide best practice for stakeholders, including patients, clinicians, health professionals, and policy makers.
Most systematic reviews include a meta-analysis, which is a statistical technique for combining (synthesising) quantitative data obtained from multiple research studies. Traditionally, most meta-analyses have used aggregate data extracted from study publications, but there is growing demand for meta-analyses that utilise individual participant data (IPD).
IPD refers to the raw (but de-identified) information recorded for each participant in a research study (e.g. a randomised trial), such as baseline characteristics, prognostic factors, treatments received, outcomes and follow-up details.
In contrast, aggregate data refers to information averaged or estimated across all participants in a particular study, such as the treatment effect estimate, the total number of participants, and the mean age and proportion of males in each treatment group. Such aggregate data are derived from the IPD, and therefore the IPD can be considered the original source material.
An example of hypothetical IPD from 10 randomised studies examining anti-hypertensive treatment is shown below:
| Prognostic factors | Time-to-event outcome | ||||||
| Study ID | Participant ID | Treatment group, (1 treatment, 0 control) |
Age at baseline, years | BMI at baseline | SBP at baseline, mmHg | Dead (1 yes, 0 no) |
Follow-up time, years |
| Study 1 | 1 | 1 | 49 | 26.02 | 176 | 0 | 5.48 |
| Study 1 | 2 | 1 | 47 | 23.60 | 148 | 0 | 5.24 |
| Study 1 | 3 | 0 | 48 | 26.03 | 144 | 1 | 0.97 |
|
[continuation] |
|||||||
| Study 10 | 337 | 0 | 35 | 25.94 | 144 | 0 | 2.92 |
| Study 10 | 338 | 0 | 40 | 24.34 | 164 | 0 | 4.99 |
| Study 10 | 339 | 1 | 45 | 27.35 | 141 | 0 | 3.69 |
IPD meta-analysis projects began to emerge in the late 1980s and early 1990s, originating mainly in cancer and cardiovascular disease fields. Calls to support IPD meta-analyses grew strongly throughout the 1990s alongside the formation of methodology working groups, in particular the Cochrane IPD Meta-analysis Methods Group.
In the decades since, the number of IPD meta-analysis projects have risen sharply.
| Figure X Number of published IPD meta-analysis articles over time, based on a crude search* in PubMed |
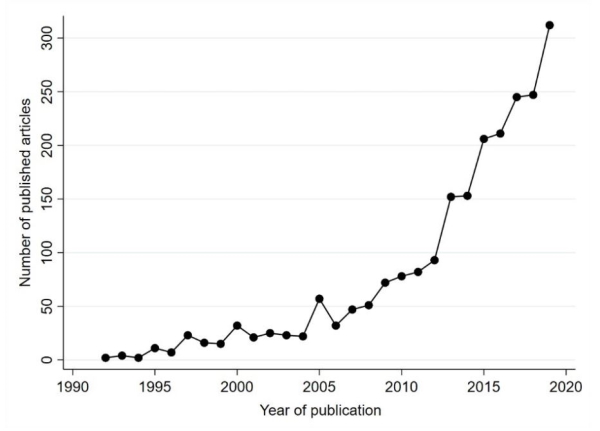
|
| * From searching for the following keywords in the Title or Abstract of the article: (meta-analysis AND individual patient data) OR (meta-analysis AND individual participant data)) OR (meta-analysis AND IPD). Source: Richard Riley |
The growth of IPD meta-analysis projects reflects their potential to revolutionise healthcare research, especially as they align with three major contemporary initiatives: reducing research waste, data sharing, and personalised healthcare.
Sharing of IPD meta-analysis maximises the contribution of existing data from millions of research participants, and so it is becoming an increasingly frequent stipulation of research funding.
IPD meta-analysis projects also allow a far broader and detailed set of analyses and research questions to be addressed than when using published aggregate data. In particular, as IPD usually increases power and ability to examine participant-level relationships, it allows a more reliable evaluation of how participant-level characteristics are associated with outcome risk and response to treatment.
IPD meta-analysis projects offer many advantages over the conventional aggregate data approach, as they can:
- improve the quantity and quality of data
- help standardise outcome and covariate definitions across studies
- enable data checking and independent scrutiny
- produce more flexible and sophisticated analyses than are possible with only existing aggregate data
- address subgroups or participant-level associations; for example, IPD are vital for a thorough investigation of treatment effect modifiers (treatment-covariate interactions), tailoring diagnostic strategies, identifying risk and prognostic factors, and individualising risk prediction
However, IPD meta-analysis projects generally require more resources than a traditional aggregate data meta-analysis, including additional costs, time and expertise.
Given the additional resources required, it is important to consider when an IPD project is needed. This depends on the particular research question, and whether IPD would produce a more reliable and comprehensive answer than using published aggregate data.
It is useful to first undertake a review to identify existing studies and whether they report suitable aggregate data to answer the research question of interest. Where focus is on overall treatment effects, published aggregate data may suffice; however, when going beyond the overall effect, IPD meta-analysis projects are usually required.
Even when IPD meta-analysis projects are needed, the available IPD needs to be of sufficient quality, record the required participant-level characteristics and outcomes of interest, and have reasonable statistical power to address the research question(s) reliably.
- Riley RD, Tierney J, Stewart LA (Eds). Individual Participant Data Meta-Analysis: A Handbook for Healthcare Research. Wiley 2021 (in-press)
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: conduct, rationale and reporting. BMJ 2010;340:c221
- Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993;341:418-22.
- Oxman AD, Clarke MJ, Stewart LA. From science to practice. Meta-analyses using individual patient data are needed. JAMA 1995;274:845-6.
- Stewart LA, Clarke MJ, on behalf of the Cochrane Working Party Group on Meta-analysis using Individual Patient Data. Practical methodology of meta-analyses (overviews) using updated individual patient data. Statistics in Medicine 1995;14: 2057-2079.
- Riley RD, Debray TPA, Fisher D, Hattle M, Marlin N, Hoogland J, et al. Individual participant data meta-analysis to examine interactions between treatment effect and participant-level covariates: Statistical recommendations for conduct and planning. Stat Med. 2020;39:2115-37.
- Simmonds M, Stewart G, Stewart L. A decade of individual participant data meta-analyses: a review of current practice. Contemporary Clinical Trial 2015;45:76-83.
- Tierney JF, Vale C, Riley R, Tudur Smith C, Stewart L, Clarke M, et al. Individual participant data (IPD) meta-analyses of randomised controlled trials: guidance on their use. PLOS Med 2015;12:e1001855.


