Biography
Employment history
Professor of Neural Tissue Engineering, School of Medicine (Faculty of Medicine and Health Sciences), Keele University (2015- )
- Lead for Medical Intercalated Degrees & Co-Lead - ASPIRE (formerly INSPIRE) medical research programme (established with support from North Staffordshire Medical Institute, Academy of Medical Sciences & Wellcome Trust.
- Director of Internationalisation, School of Medicine.
Senior Lecturer School of Medicine (Faculty of Medicine and Health Sciences), Keele University (2011-14)
Lecturer, School of Medicine (Faculty of Medicine and Health Sciences), Keele University (2007-11).
Junior Fellow-Multiple Sclerosis Society, UK, Cambridge Centre for Brain Repair and Veterinary Medicine, Cambridge University (2003-07)
Research Associate, Cambridge Centre for Brain Repair and Veterinary Medicine, Cambridge University (Wellcome Trust funded) (2000-03)
D.Phil “Cell Genesis in Hamster Cortex”, Oxford University, Wadham College, 2000, funded by an international scholarship.
MSc Medical Physiology All India Institute of Medical Sciences (AIIMS), New Delhi, 1996
BSc (Hons) in Human Biology (Physiology) AIIMS, New Delhi, 1994
Research and scholarship
Research group: Neural Tissue Engineering Keele (NTEK)
Research interests
- Stem cell sprays for neurological injuries.
- Development of electroactive hybrid materials for neural repair.
- Use of novel biomedical engineering strategies such as magnetic nanoparticles, implantable hydrogels and neural cell seeded polymer scaffolds to promote neural regeneration.
- Development of in vitro neural injury models to reduce reliance on live animal experiments.
- Investigation into the molecular effects of immunotherapies on neural development.
We collaborate widely and currently use a combination of methods for these studies including:
- 3D printing and bioelectronics with Dr Shery Huang and Prof George Malliaras, Cambridge Nanoscience Centre.
- Isolated neural cell culture (primary cultures of neural stem cells, oligodendrocyte precursor cells, olfactory ensheathing cells, oligodendrocytes, astrocytes and neurons).
- Microarray, proteomic and bioinformatic analysis of drug and nanoparticle treated neural cells for safety testing (with Prof Richard Emes, Director Advanced Data Analysis centre, Nottingham University; Dr Sarah Hart, Keele Proteomics unit).
- Electrophysiological recordings from neural cells grown in implantable, neuromimetic matrices (with Dr Michael Evans, Keele University).
- TEM to monitor intracellular nanoparticle fate, and development of novel, high throughput and high resolution SEM methods to examine neural cell-nanomaterial interactions (with Prof David Furness, Director, Keele University Electron Microscopy Unit).
- Testing and characterising novel nanoparticles for magnetic resonance imaging and gene delivery in neural transplant populations (with Dr Boris Polyak, Drexel University; Dr Humphrey Yiu, Heriot Watt University, Prof Jean Paul Llellouche, Bar Ilan University, Israel; Prof Harish Poptani, Liverpool University Preclinical Imaging Centre and nanoTherics Ltd) used with state of the art static and oscillating field ‘magnetofection’ techniques.
- In vivo models of CNS injury in the adult and neonatal brain and spinal cord (demyelination and transection) with cell transplantation.
- Testing functionalised implantable materials to promote regeneration in neurological injury (with Dr Nicolas Granger, Bristol University, Dr Paul Roach, Keele University, Prof Robin Franklin, Cambridge University and BioGel X Ltd).
- Development of in vitro models of neurological injury models (including human tissue) to reduce reliance on live animal experiments (with Dr Niko Tserakis and Mr Jon Sen, Department of Neurosurgery, University Hospital of North Midlands).
 |
|
Journal covers from the Chari laboratory. MNP labelled, transplanted astrocytes integrated into organotypic cerebellar slice circuitry (Top left). Glucocorticoid receptor expression in neural cells (Top middle). Purified oligodendrocytes used for magnetic nanoparticle based transfection (Top right). Schematic of myelin genesis and influence of corticosteroids (Bottom left). MNP mediated gene delivery in a multicellular neural model (Bottom middle). Glucocortocoid receptor expression in neural stem cells and their progeny (bottom right). |
 |
 |
|
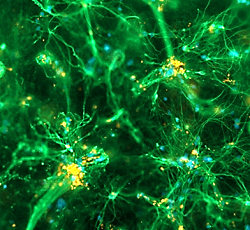
Dense 3-D network of magnetic nanoparticle (red) labelled strocytes (green) growing through the depth of a hydrogel, 37 days of cell growth intragel, photo Jacki Tickle |
Rodent neural stem cells genetically engineered using nanoparticles functionalised with minicircle DNA vectors, photo Alinda Fernandes |
 |
 |
|
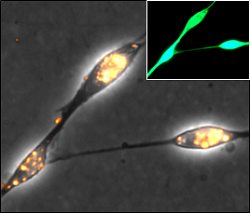
Canine olfactory ensheathing cells (transplant population) genetically engineered using functionalised magnetic nanoparticles. Target protein (green), photo Alexander Delaney |
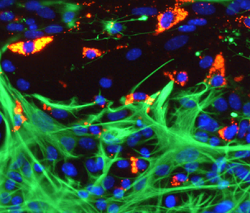
Microglia in a spinal cord slice transecting injury site showing avid loading and competitive uptake of nanoparticles (red) versus slice astrocytes (green). Nuclei in blue, photo Alan Weightman |
|
|
|
|
Reduction in EdU labelled proliferating cells (green) in corticosteroid treated neurospheres versus vehicle treated control spheres (left). Nestin (red) and DAPI (blue), photo Rawaa Saleem Al Mayyahi |
|
 |
 |
|
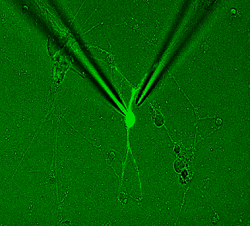
Single cell electrical recording from a nanoengineered primary cortical neuron, photo Arwa Shakli and Mike Evans |
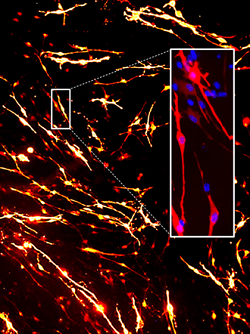
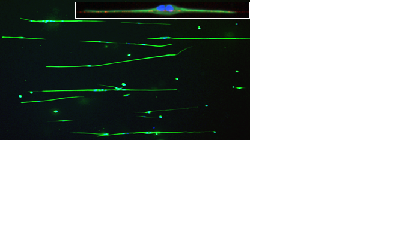
Astrocytes aligning on polymer nanofibres embedded in hydrogel, photo Divya Chari |
Further information
External roles:
- Member-Medical Research Council Developmental Pathway Funding Scheme, 2021-2026
- Member- The Expert Review Network (TERN), UK Multiple Sclerosis Society, 2023-
- Core Member - Committee C (Genes, development and STEM* approaches to biology), British Biotechnology and Biological Sciences Research Council (2017-2022).
- Member - Associate Peer Review College, Engineering and Physical Sciences Research Council.
- Member - Grants Advisory Committee, International Spinal Research Trust.
- Member - Pool of experts, British Biotechnology and Biological Sciences Research Council (2015-17).
- Member - Editorial Board, Midlands Medicine.
Publications
Projects and funding
2023-25 British Council Going Global Partnerships grant– UK-China Institutional Partnership Exploration Fund with Shandong First Medical University China (to Keele School of Medicine)
2023 Enhancing high-throughput neurophysiology research through multiwell, multielectrode array capability, Co-investigator with Principal Investigator Dr Chris Adams, Keele, Medical Research Council equipment award
2023 Manipulating Ependymoma Oncogenic Response Using Light-Induced Engineering and Stem Cells. Principal Investigator Vinoj George, Co-investigators DM Chari and R Rahman (University of Nottingham), The Humane Research Trust
2022 Association of Clinical Pathologists intercalation award for medical intercalator in group
2022 GlycoMatrix: Engineering Tuneable Stem Cell Niches Enhanced with Glycosaminoglycan Instructive Cues. EPSRC New Horizons award, Co-investigator with J Turnbull, (Keele)
2021 UKRI World Class Laboratories funding for microscopy facility refurbishment (with Professor
2020 Royal College of Physicians Wolfson clinical intercalation award to medical intercalator in group
2019 Academy of Medical Sciences (AMS)/Wellcome Trust INSPIRE grant to fund medical student research till 2022 with BioNow, Birmingham and Warwick Universities
2019 EPSRC Healthcare Technologies Discipline Hopper Grant "A new technology platform for neuro-regeneration: Next generation electroactive bioprostheses for spinal cord injury (SCI)" with Dr Shery Huang and Prof George Malliaras, Cambridge University
2019 Faculty of Natural Sciences Research Development Fund “Developing a stem cell spray for traumatic brain injury”, with Dr Chris Adams, Keele University
2018 EPSRC Centre for Doctoral Training PhD studentship "Developing 3D in vitro models of traumatic neurological injury for stem cell imaging" Primary supervisor
2017 Faculty of Natural Sciences PhD studentship “Investigating the chick embryo as a neurological injury model for screening new nanotherapies” (co-supervisor with Primary supervisor Dr Chris Adams)
2017 Association of Clinical Pathologists and Wolfson Foundation (Royal College of Physicians) funding for Clinical Intercalated MPhil "Evaluating the feasibility of using neurosurgical hydrogel materials as protective delivery systems for stem cell transplant populations" Primary supervisor (co-supervisors Mr Niko Tzerakis and Mr Jon Sen, Neurosurgery Department, UHNM)
2017 MRC Confidence in Concept funding "Protective matrices for delivery of genetically augmented canine olfactory ensheathing cell transplants" Principal investigator (with Dr Chris Adams, Keele University; Dr Nicolas Granger, Royal Veterinary College London; Prof Nicolas Jeffery, Texas A& M University and Mr Niko Tserakis, UHNM Neurosurgery department)
2016 Langford Trust PhD studentship, “Developing tissue matched implantable hydrogels for delivery of olfactory ensheathing cells into sites of spinal cord injury”, co-applicant with Dr Nicolas Granger and Dr Liang Fong Wong, Bristol University
2016 Clinical MPhil "Building 3D models of the neural immune system with live cell imaging to test cell scavenger function" Primary supervisor
2016 PhD studentship "Investigating the neural cell specificity and immune evasion of novel cell penetrating peptides for neurological targeting" Primary supervisor
2016 Natural Sciences Faculty support fund "An electrophysiological investigation into the properties of nano-engineered neurons propagated on ‘neuromimetic ‘materials" Co-investigator (PI Dr Mike Evans, Keele Faculty of Natural Sciences)
2016 North Staffordshire Medical Institute charity funding "Assessing human brain slices as ‘benchtop’ injury models" Principal Investigator (with Dr Niko Tzerakis, Department of Neurosurgery, University Hospital of North Midlands)
2015 Comparative Clinical Science Foundation and Wolfson Foundation (Royal College of Physicians) funding for Clinical Intercalated MPhil "Developing tissue matched implantable hydrogels for delivery of olfactory ensheathing cells into sites of spinal cord pathology in domestic dogs" Primary supervisor (Co-supervisor Nicolas Granger, Bristol University)
2015 Keele School of Pharmacy funding "Investigating the interaction of metabolic regulatory molecules with cells of the central nervous system" Co-investigator
2015 EPSRC E-TERM Landscape Fellowship "Multicellular and neuromimetic hydrogels as implantable constructs to enhance repair in spinal cord injury"
2015 PhD studentship "Developing advanced implantable materials for neurological injury" Primary supervisor
2014 PhD studentship "Interface of nanotechnology platforms and neuronal cells" Primary supervisor
2014 EPSRC Centre for Innovative Manufacturing in Regenerative Medicine medical research studentships "Can magnetic hyperthermia safely enhance magnetic nanoparticle labelling of neural stem cells?" and "Using a novel multifunctional magnetic nanoparticle with a state-of-the-art 'magnetofection' device to enhance gene transfer to neural transplant cells" Primary supervisor, with industrial partners nanoTherics Ltd.
2014 MMed Sci clinical intercalation project "Investigating physical microenvironments of neural cells to develop neuro-mimetic materials- An Atomic Force and Electron Microscopy study" Primary supervisor
2014 PhD studentship "Evaluating the effects of immunotherapies on precursor/stem cells of the central nervous system" Primary supervisor
2013 PhD studentship"The Role of Nicotinamide on Directed Stem Cell Differentiation to Neuronal Fates in vitro". Co-supervisor
2013 Clinical MPhil "An investigation of parameters that govern the uptake of medically relevant magnetic nanoparticles in primary neural stem cells" Primary Supervisor
2012 PhD studentship "Evaluation of astrocyte interactions with nanomaterials" Primary supervisor
2012 EPSRC 3ME Initiative- Bridging the Gaps “Mathematical modelling of magnetic particle uptake in neural cell populations: Can we predict modes of particle handling in complex cell systems?” Principal Investigator
2012 BBSRC Project Grant “Magnetic nanoparticle mediated delivery of neurotherapeutic genes to multipotent neural stem cell transplant populations” Principal Investigator
2012 EPSRC E-TERM Landscape Fellowship "Magnetic nanoparticle based transfection of oligodendrocyte precursor cells for transplantation into a 'living brain' reductionist demyelination model", (www.regener8.ac.uk/)
2012 ISTM capital bid funding to purchase (1) Stereomicroscope with imaging system, (with ISTM neuroscience theme research scientists) ; and (2) Electrophysiology rig for electrical recordings from acute brain slices and organotypic slice culture injury models (with Dr David Mazzocchi Jones and Dr Mike Evans).
2011 MMed. Ed. project " A survey of intercalation at UK medical schools" Co-supervisor
2011 EPSRC Doctoral Training Centre PhD studentship “Strategies to enhance magnetic nanoparticle mediated delivery of therapeutic biomolecules to neural transplant populations ” Primary Supervisor
2010 North Staffordshire Medical Institute grant “Transcriptional effects of corticosteroid (CS) therapy on myelin genesis and neuronal health” Principal Investigator
2010 EPSRC Doctoral Training Centre PhD studentship “Development of Novel Tissue Engineering Strategies for Spinal Cord Repair” Primary Supervisor
2009 Royal Society UK research grant “Magnetic nanoparticle MNP mediated gene transfer to oligodendrocyte progenitor cells (OPCs) derived for transplantation” Principal Investigator
2008 United States National Multiple Sclerosis Society Pilot Award “Can magnetic nanoparticles be used to modulate gene expression in demyelinated areas?” Principal Investigator
2008 BBSRC New Investigator Award “Development of magnetic nanoparticle (MNP) based delivery system for gene transfer to multipotent neural precursor cells (NPCs)” Principal Investigator
2007 British Neuropathological Society Small Grant “Effects of corticosteroid (CS) administration on myelination in the developing central nervous system (CNS)” Principal Investigator
2007 Keele School of Medicine startup fund &PhD studentship “Applications of magnetic nanoparticles for oligodendrocyte precursor cell transplantation strategies” Primary Supervisor
2003 UK Multiple Sclerosis Society, Junior Research Fellowship (four-year) “Effects of corticosteroid administration on oligodendrocyte progenitor cells and remyelination”
1996 Felix Scholarship to undertake DPhil studies at Oxford University
School address:
School of Pharmacy and Bioengineering
Hornbeam Building
Keele University
Staffordshire
ST5 5BG
Research centre address:
School of Pharmacy and Bioengineering
Guy Hilton Research Centre
Thornburrow Drive
Stoke-on-Trent
ST4 7QB
Tel: +44 (0) 1782 674988
Undergraduate enquiries:
Email: enquiries@keele.ac.uk
Tel: +44 (0)1782 734010
Postgraduate enquiries:
Please contact the CPD4ALL team:
Email: phab.postgraduate@keele.ac.uk
Keele Centre for Medicines Optimisation (KCMO)
Tel: +44 (0)1782 733831 / 734131
The Virtual Patient project enquiries:
Contact our Digital Development team:
Email: pharmacy.digital@keele.ac.uk



