Structural Biology
The Structural Biology Research group (Professor Trevor Greenhough, Dr Annette Shrive) aims to characterise the molecular mechanisms by which molecules of the innate immune system recognise and bind to their natural targets and effect clearance through interaction with components of immune system pathways.

This structural immunology includes ligand/pathogen recognition by the pentraxins CRP and SAP from human and a variety of other sources including Limulus, rat and fish; by collectins including hSP-D, SP-A, CL-46 and conglutinin and by other proteins targeted by our collaborators for study. Of particular interest in the group is the pathogen-host interaction, with a particular emphasis on humans and mammals but also extending through to fish and to plants. In addition to crystallography, our structural investigations utilise complementary techniques, both in-house and with collaborators, including SRCD, cryoEM, mass spectrometry, carbohydrate-type ligand manipulation and analysis and clinical measurement
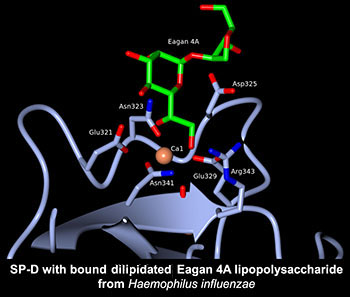 Human lung surfactant protein D (hSP-D) can directly interact with carbohydrate residues on pulmonary pathogens and allergens, stimulate immune cells, and manipulate cytokine and chemokine profiles during the immune response in lungs. The high resolution crystal structures, both native and ligand bound, of a therapeutically active recombinant fragment of SP-D define the fine detail of the mode and nature of carbohydrate recognition and provide insights into how a small fragment of human SP-D can bind to whole pathogens and at the same time recruit and engage effector cells and molecules of humoral immunity. Particular current emphasis (in collaboration with University of Southampton and MRC Mammalian Genetics Unit Harwell) is on recognition of native and mutant strains of bacterial lipopolysaccharide and is complemented by in-house collaborations on carbohydrate analysis and manipulation with Dr Mark Skidmore and Dr Gavin Miller.
Human lung surfactant protein D (hSP-D) can directly interact with carbohydrate residues on pulmonary pathogens and allergens, stimulate immune cells, and manipulate cytokine and chemokine profiles during the immune response in lungs. The high resolution crystal structures, both native and ligand bound, of a therapeutically active recombinant fragment of SP-D define the fine detail of the mode and nature of carbohydrate recognition and provide insights into how a small fragment of human SP-D can bind to whole pathogens and at the same time recruit and engage effector cells and molecules of humoral immunity. Particular current emphasis (in collaboration with University of Southampton and MRC Mammalian Genetics Unit Harwell) is on recognition of native and mutant strains of bacterial lipopolysaccharide and is complemented by in-house collaborations on carbohydrate analysis and manipulation with Dr Mark Skidmore and Dr Gavin Miller.
Key references:
- Paterson et al. (2019) Journal of Biological Chemistry, 294, 17155-17165.
- Littlejohn et al. (2018) PLoS One. 18;13(6):e0199175.
- Clark et al. (2016) Infection and Immunity. 84, 1585-1592.
- Shrive et al. (2009) Journal of Molecular Biology. 394, 776-788.
- Kishore et al. (2004) Immunology Letters 95, 113-128.
- Kishore et al. (2004) Trends in Immunology 25, 551-561.
- Shrive et al. (2003) Journal of Molecular Biology. 331, 509-523.
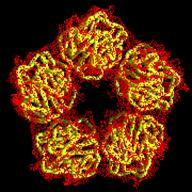
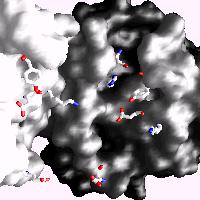
Human C-reactive protein (CRP) is a trace plasma protein which exhibits rapid increases in concentration of up to 1000-fold in response to tissue damage and inflammation. As the classical acute-phase reactant, CRP is used almost universally as a clinical indicator of inflammation and underlying infection. The host defence functions of human CRP depend to a great extent on its ability to activate the classical complement pathway and a major current interest is in structural insight into its ligand recognition and effector function using a variety of complementary techniques (crystallography, EM, SRCD, MS). Alongside this work we are interested in the effects of CRP from a clinical perspective and are currently investigating possible modified forms of C-reactive protein in patient serum samples (ethical approval REC reference 17/WM/0150), in collaboration with Professor Tony Fryer and Dr Sarah Hart. For further information on this study please contact Dr Annette Shrive (a.k.shrive@keele.ac.uk) or Professor Tony Fryer (Anthony.Fryer@uhnm.nhs.uk).
Key references:
- Williams et al. (2020) Frontiers in Immunology, 11, 115.
- Shrive et al. (1996) Nature Struct. Biol. 3, 346-354.
- Agrawal et al. (2001) J. Immunol. 166, 3998-4004.
- Ramadan et al. (2002) Acta Cryst. D58, 992-1001.
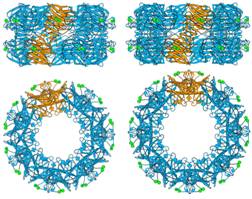
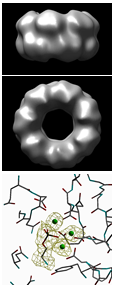
Our work on the serum-amyloid-P-component-like pentraxin from Limulus polyphemus has involved the isolation, sequencing and structure of the doubly-stacked octameric structure of the previously undiscovered pentraxins, the first structure of an invertebrate lectin-pentraxin. We have also carried out complementary studies (in collaboration with University of California) of a new molecular form containing doubly stacked heptamers, and reported the native and PE-bound structures.
References:
- Shrive et al. (2009) J. Mol. Biol. 386, 1240-1254.
- Tharia et al. (2002) J. Mol. Biol. 316, 583-597.
- Shrive et al. (1999) J. Mol. Biol. 290, 997-1008
Our work on C-reactive protein (CRP) in carp with Prof Dave Hoole, as part of two successive EU Marie Curie training networks with European partners, seeks to establish the role of CRP in fish and its potential use as a biomarker of health in a similar manner to the use of CRP in humans in the clinical setting. Specific projects on carp – the most economically important cultured fish in the world - include extraction, purification and characterisation of fish immune and acute-phase proteins, the potential for immune modulation in warm-water fish by feeding of glucans, and the impact of pollution on fish immunity.
Key references:
- Pionnier et al. (2014) Fish and Shellfish Immunology 39, 285-295.
- Pionnier et al. (2013) Fish and Shellfish Immunology 34, 819-831.
- MacCarthy et al. (2008) Developmental and Comparative Immunology 32, 1281-1289.
- Cartwright et al. (2004) Developmental and Comparative Immunology 28, 113-125.
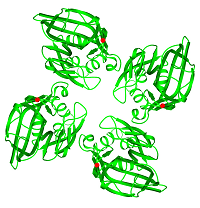
FIBCD1 (a collaborative project with the University of Southern Denmark) is a tetrameric plasma membrane protein that uses a fibrinogen-like recognition domain (FReD) for pattern recognition of acetyl groups on chitin. The x-ray structures of the FIBCD1 FReD reveal how FIBCD1 binds acetylated pathogen associated molecular patterns (PAMPS) and endogenous glycosaminoglycans, combining versatility with conservation to recognise its targets.
Key reference:
- Shrive et al. (2014) J. Biol. Chem. 289, 2880-2887.

